Introduction
Venetoclax is an oral targeted therapy drug that is FDA-approved for the treatment of chronic lymphocytic leukemia and small lymphocytic lymphoma (CLL/SLL) in both the frontline and relapsed/refractory (R/R) settings. While Venetoclax's dominant mechanism of action is the inhibition of Bcl-2 and thus, priming malignant CLL/SLL cells for apoptosis, there are emerging data highlighting the effects of Venetoclax on T-cell fitness/function. T-cell populations in CLL/SLL are known to be functionally impaired, in part due to abnormal expansion of T-regulatory cell (Treg) populations that blunt normal anti-tumoral cellular immune responses by CD8 + T-cells, Th1 Helper T-cells, and NK-cells and allows CLL/SLL cells to proliferate. Treg cell frequency has also been shown to directly correlate with CLL/SLL progression, and treatment of CLL/SLL results in lower circulating Treg populations. The unfavorable and generally anti-inflammatory tumoral microenvironment fostered by CLL/SLL cells is a major obstacle to the development of effective chimeric antigen receptor (CAR) T-cell options for this disease, for which there are currently no FDA-approved products despite more than a decade of investigation.
Objective: In our current work, we studied the impact of Venetoclax on T-cell composition and function in serially collected specimens from patients with CLL/SLL; and evaluated the function of CAR T-cells manufactured from Venetoclax treated patients.
Methods
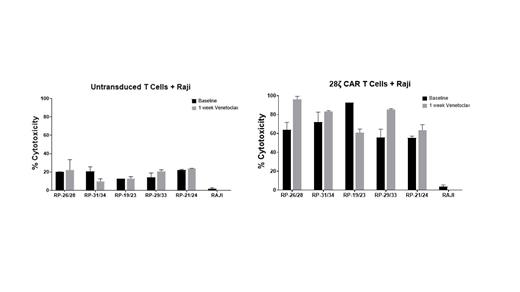
Peripheral blood mononuclear cells (PMBCs) and plasma was collected from CLL/SLL patients (N=5) who were treated with Venetoclax using the standard ramp up dosing-protocol. All procedures were approved by our IRB under BDR 164122. Samples were collected at pre-treatment (baseline) and several post-Venetoclax dosing timepoints (Day +1 day, day +7, and day +30). B-cells were isolated by negative selection using magnetic beads. CLL cells were the B-cells were exposed Venetoclax alone or in combination with several chemotherapy agents. Cell viability was determined by Cell Titer glo after 48hrs of drug exposure. In addition, PBMCs samples were analyzed using multicolor parameter flow cytometry to determine T-cell subsets composition including memory phenotype/stemness and others at each time point. Thorough serum cytokine (IL-15, IL-6, Il-2 and IL-10) analysis was conducted at each time point. Using baseline and 1-week post Venetoclax PBMCs samples, T-cells were activated, and autologous second generation CD19-directed CAR T-cells were manufactured. The efficacy of CARs were then tested at a 1:1 effector to target ratio against Raji and Raji 4RH cells for 24 hours and analyzed for cytotoxicity via flow cytometry.
Results
As expected Venetoclax was active in all samples tested. In addition, synergistic activity was observed when combined with chemotherapy agents or targeted agents (i.e. BRD-4 inhibitors). Regarding T-cell function, flow cytometry analysis at day +30 of Venetoclax therapy demonstrated that T-helper and T-cytotoxic T-cells displayed less exhaustion markers when compared to pre-Venetoclax samples (Baseline). Of interest, autologous αCD19 CAR T-cells manufactured 1 week following Venetoclax therapy induced higher cytotoxicity against Raji (rituximab chemotherapy sensitive cell lines) or Raji 4RH (rituximab-chemotherapy resistant cell lines) cells when compared to CAR T-cells generated from Venetoclax pre-treatment T-cells.
Conclusion
Our data suggests that Venetoclax has anti-tumor activities beyond direct CLL cytotoxicity. Venetoclax therapy appears to repair T-cell subsets in CLL patients. Perhaps by decreasing exhaustion markers in CD4/CD8 T-cells, Venetoclax potentiate the cytotoxicity of anti-CD19 CAR T cells against B-cell lymphoma. Further work is needed to fully elucidate the mechanism(s) by which Venetoclax modifies the fitness and function of T-cell subsets. These efforts may guide the development of combination strategies using BH-3 mimetics and T-cell engagers based therapeutic options for patients with CLL/SLL.
Disclosures
Hernandez-Ilizaliturri:BMS: Consultancy; Kite: Consultancy; Gilead: Consultancy; AbbVie: Consultancy; BioGene: Consultancy; Novartis: Consultancy; Epizyme: Consultancy; Dava Oncology: Consultancy; Amgen: Consultancy; ADC Therapeutics: Consultancy; Incyte/Morphosys: Consultancy; Collectar: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal